Introduction:
EUS-guided gastroenterostomy is an emerging technique for the palliation of malignant and, most recently, benign gastric outlet obstruction (GOO). The most frequent causes of GOO are malignant neoplasms located at the pancreas, gallbladder, common bile duct, and distal stomach. So far, laparoscopic gastroenterostomy has been considered the gold standard palliative treatment for GOO, offering long-lasting relief of the obstruction but with the disadvantage of longer hospital stay and surgery-related adverse events. Endoscopic placement of gastroduodenal metallic stents is a valid alternative to surgical treatment, offering almost immediate relief of the GOO with the disadvantage of a higher rate of obstructive symptoms due to stent occlusion 2–3 months later. The invention of lumen-apposing metal stents (LAMSs) has made possible the EUS-guided creation of GI anastomoses, particularly EUS-guided gastroenterostomy (EUS-GE). Newer data suggest that EUS-GE could combine the advantages of surgical treatment and endoscopic enteral stenting, providing fast and long-lasting relief of GOO with a reduced adverse events rate. Most data are from expert centers and therefore, prior to widespread adoption, high quality randomized controlled studies are desirable.
Do’s and Don’ts:
- Correct patient selection is a key factor for the clinical success of EUS-GE. GOO is commonly a late event in the natural course of the above-mentioned malignancies. A patient with a very poor performance status (e.g., ECOG 3 or 4) may not benefit from any invasive measure, and the approach to therapy and intervention should preferably be determined by a multidisciplinary tumor board.
- From the technical aspect, partial gastrectomy and malignancy involving the proximal stomach are contraindications to EUS-GE. The presence of multiple sites of enteral strictures (e.g., diffuse peritoneal carcinomatosis) precludes clinical benefit of EUS-GE. In this situation, a well-documented endoscopy and computed tomography (CT) scan are advisable.
- EUS-GE is an advanced endoscopic intervention in which CO2 insufflation, general anesthesia and fluoroscopic guidance are all necessary for the safety of the procedure.
- Preference should be given to a large-diameter (15–20-mm) LAMS built into a cautery enhanced fistula creation device (“hot” delivery system). This reduces the number of steps and increases the chances of technical success.
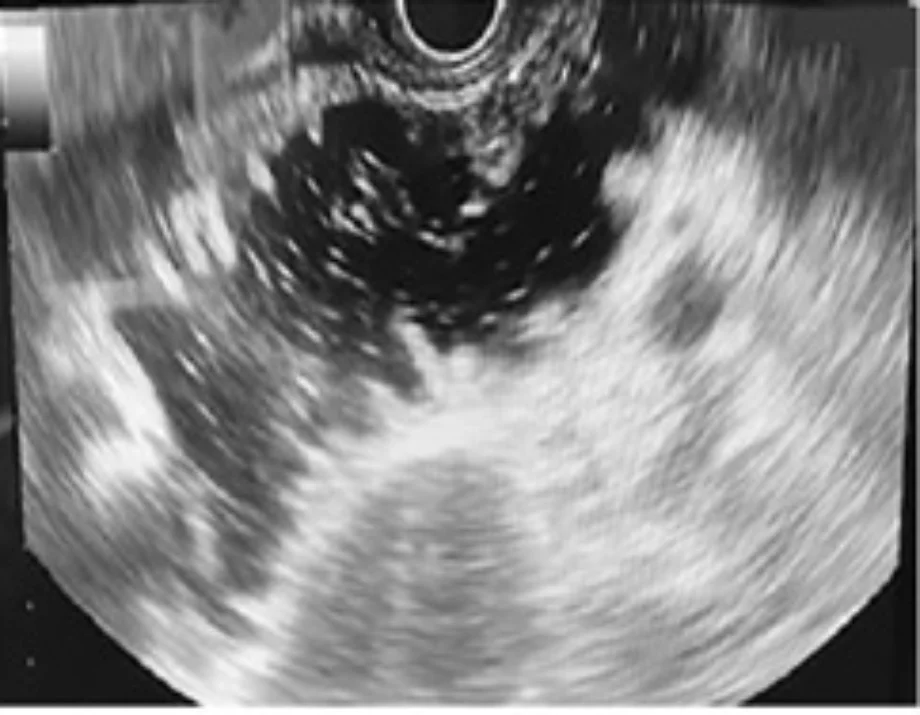
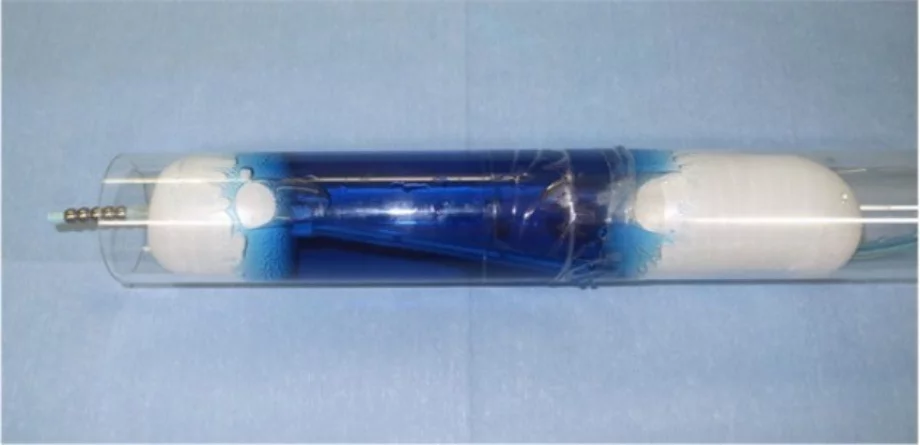
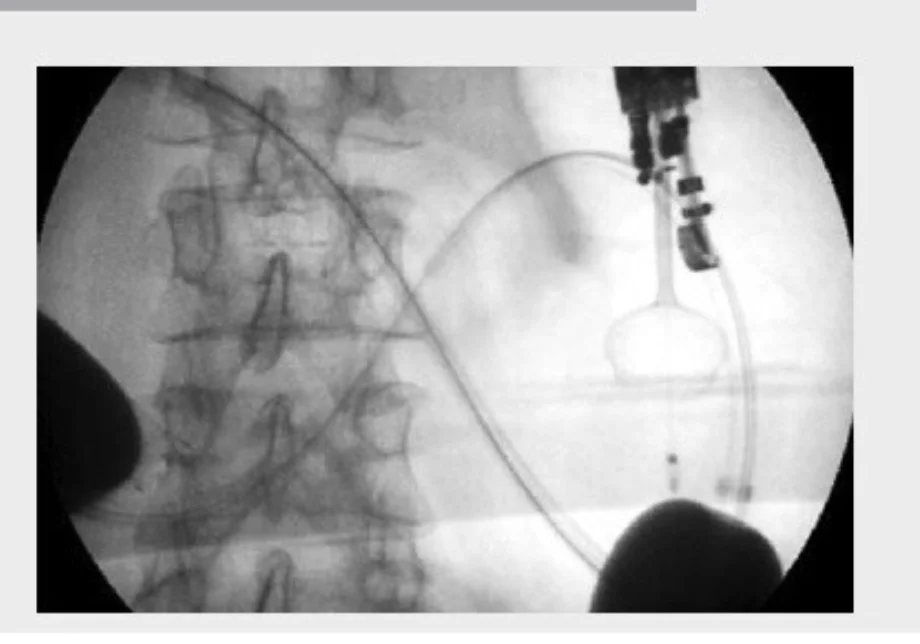
- EUS-GE is an evolving technique. There are at least four different ways to perform it. In most of them, it is crucial to obtain a well-distended, fluid-filled small-bowel loop next to the posterior wall of the stomach (Figure 1). The use of the double-balloon occluder (Figure 2) developed by Takao Itoi facilitates the distension of the small-bowel loop. If that is not available, a 7-Fr catheter (e.g., the pusher of a 7-Fr plastic biliary stent) should be advanced over the guidewire beyond the point of gastroduodenal obstruction and into the distal duodenum. The use of a 50–60-mL syringe or a dedicated pump facilitates the quick infusion of 400–500 mL of saline (or sterile water) solution, along with 5–10 mL of 2% indigo carmine solution, into the distal duodenum. A prone position of the patient may facilitate retention of the fluid within the small-bowel lumen. If the double-balloon occluder is available, the distal and proximal balloons are filled with 35–40 mL of a diluted contrast solution, under fluoroscopic guidance (Figure 3). The EUS scope is advanced into the proximal stomach and visualization of the distended small-bowel loop is possible. After advancement of the echoendoscope into the stomach, further injection of the saline + dye solution can be done and this can be visualized under EUS guidance, guaranteeing that this is the correct small-bowel loop and not a colonic segment. Intravenous antispasmodics (e.g., scopolamine or glucagon) can be useful for interrupting small-bowel peristalsis during the procedure.
EUS view of distended fluid-filled small bowel loop next to the posterior gastric wall
Double balloon catheter designed by Prof Takao Itoi
Fluoroscopic view of the correct position of the double-balloon catheter. Note the two balloons filled with diluted contrast.
- Under EUS (and not fluoroscopic) guidance, the LAMS delivery system is introduced through the gastric and small-bowel walls. Typically, a high output, pure cut current (e.g., 100 W AUTOCUT, Erbe (Erbe Elektromedizin, GmbH, Tübingen, Germany.) is used for the desired effect. There is no need to push the delivery system too hard or to jab it into the small bowel. The technique should be similar to what we do when we perform pancreatic fluid collection drainage.
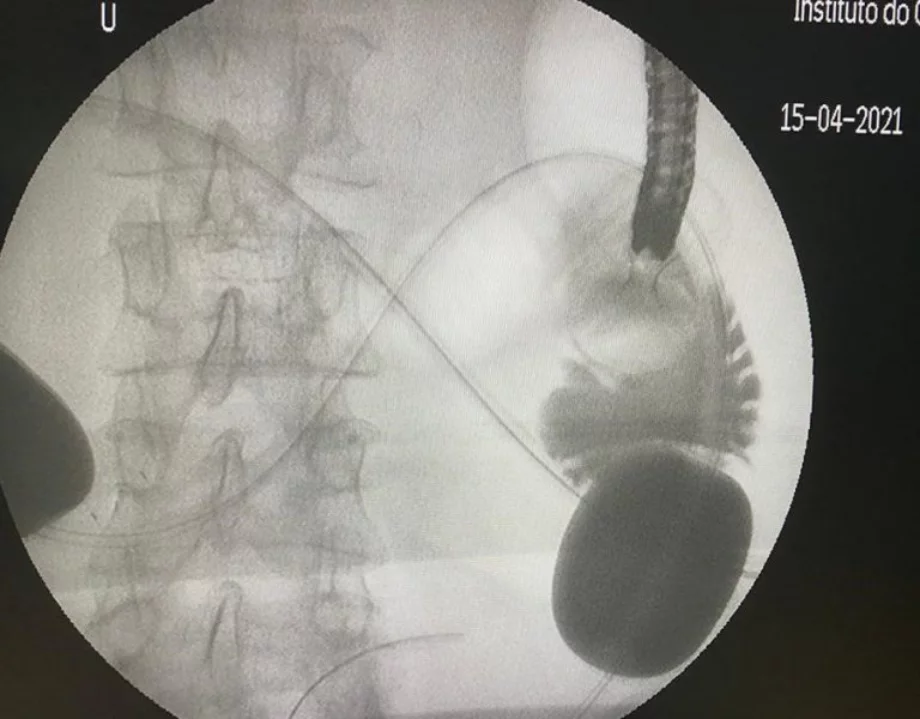
- The sequence of the stent deployment depends on the type of stent used. Generally speaking, the distal flange is deployed in the small-bowel loop and it is retracted so that it compresses the small-bowel wall against the gastric wall. At this moment, the proximal flange is deployed inside the gastric lumen. The correct position of the stent is confirmed by the reflux of the saline + dye solution in the stomach and by the direct visualization of the small-bowel folds. The stent deployment is performed under EUS control only. At the end of the procedure, we inject 40 mL of contrast under fluoroscopic guidance through the LAMS opening to confirm the free flow of the contrast from the stomach into the small-bowel loop (Figure 4). LAMS misdeployment during EUS-GE occurs in up to 10% of cases, the most frequent type being the inadvertent deployment of the distal flare in the peritoneum. It can usually be managed by removing the LAMS and closing the gastric defect with suture or clips.
Fluoroscopic view of the passage of contrast from the stomach to the small bowel through the gastroenteral anastomosis created under EUS guidance.
- Postoperatively, the patient starts a fluid diet the next day. An abdominal X-ray confirms adequate expansion of the stent on the 1st postoperative day. The diet is progressed to soft food on the 2nd postoperative day. If soft and liquid food is tolerated without vomiting, the patient can be discharged from hospital on the 3rd postoperative day.
- We recommend antibiotic prophylaxis for EUS-guided gastroenterostomy, interruption of anticoagulation for 48 h after the procedure, and standard-dose proton pump inhibitor (PPI) treatment for 2 months after the procedure.
- For patients with survival >6 months, exchange of the LAMS for a new one may be necessary.